Video Library
Learn more about VYXEOS
Learn more about VYXEOS
with our video library


Secondary aml
7:33 MINS

Speaker: Jonathan A. Abbas, MD
Managing sAML
Transcript
((Dr. Abbas)) Hello, I’m Dr Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
Today I’m going to talk about improving outcomes in patients with sAML subtypes, t-AML and AML-MRC, using VYXEOS.
An important challenge in treating AML is recognizing that sAML is a unique entity, which behaves very differently than conventional AML. Because we now have some novel treatments for sAML, it is imperative that we identify these patients to be able to give them a chance at remission. Therefore, whenever I identify an sAML patient who is a candidate for induction therapy, I always ask myself, is this patient a good candidate for VYXEOS?
Patients with sAML have historically exhibited poor survival, so selection of appropriate first-line therapy is important to achieving remission and ultimately prolonging survival.
In patients who are clinically stable and able to tolerate intensive chemotherapy, considering a treatment such as VYXEOS may improve outcomes in patients with sAML.
((Dr. Abbas)) Hello, I’m Dr Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
Today I’m going to talk about improving outcomes in patients with sAML subtypes, t-AML and AML-MRC, using VYXEOS.
An important challenge in treating AML is recognizing that sAML is a unique entity, which behaves very differently than conventional AML. Because we now have some novel treatments for sAML, it is imperative that we identify these patients to be able to give them a chance at remission. Therefore, whenever I identify an sAML patient who is a candidate for induction therapy, I always ask myself, is this patient a good candidate for VYXEOS?
Patients with sAML have historically exhibited poor survival, so selection of appropriate first-line therapy is important to achieving remission and ultimately prolonging survival.
In patients who are clinically stable and able to tolerate intensive chemotherapy, considering a treatment such as VYXEOS may improve outcomes in patients with sAML.
4:56 MINS

Speaker: Sara M. Tinsley, PhD, ARNP, AOCN
Secondary AML diagnosis
Transcript
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) It is important to differentiate between de novo AML and secondary AML because the treatment approach may differ.
We emphasize that waiting for test results is the right thing to do before beginning treatment in AML.
We may need to confirm an sAML diagnosis via the myeloid mutation panel to ensure that patients are receiving the right treatment for their AML type.
When deciding on treatment, we look at the patient’s performance status as well as their stability and fitness, but not so much their age, unless they are in their eighties.
We assess if they would be fit for chemo, which may still be possible, even at that age.
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) It is important to differentiate between de novo AML and secondary AML because the treatment approach may differ.
We emphasize that waiting for test results is the right thing to do before beginning treatment in AML.
We may need to confirm an sAML diagnosis via the myeloid mutation panel to ensure that patients are receiving the right treatment for their AML type.
When deciding on treatment, we look at the patient’s performance status as well as their stability and fitness, but not so much their age, unless they are in their eighties.
We assess if they would be fit for chemo, which may still be possible, even at that age.


vyxeos clinical information
10:29 MINS
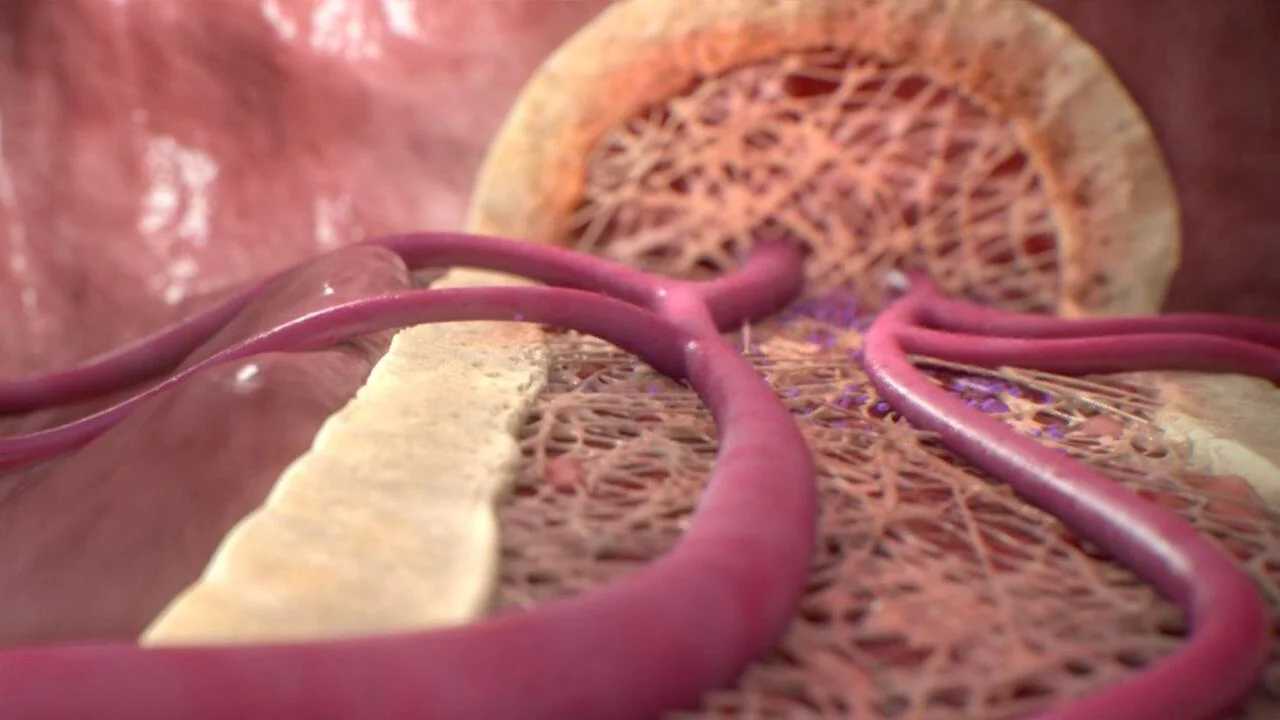
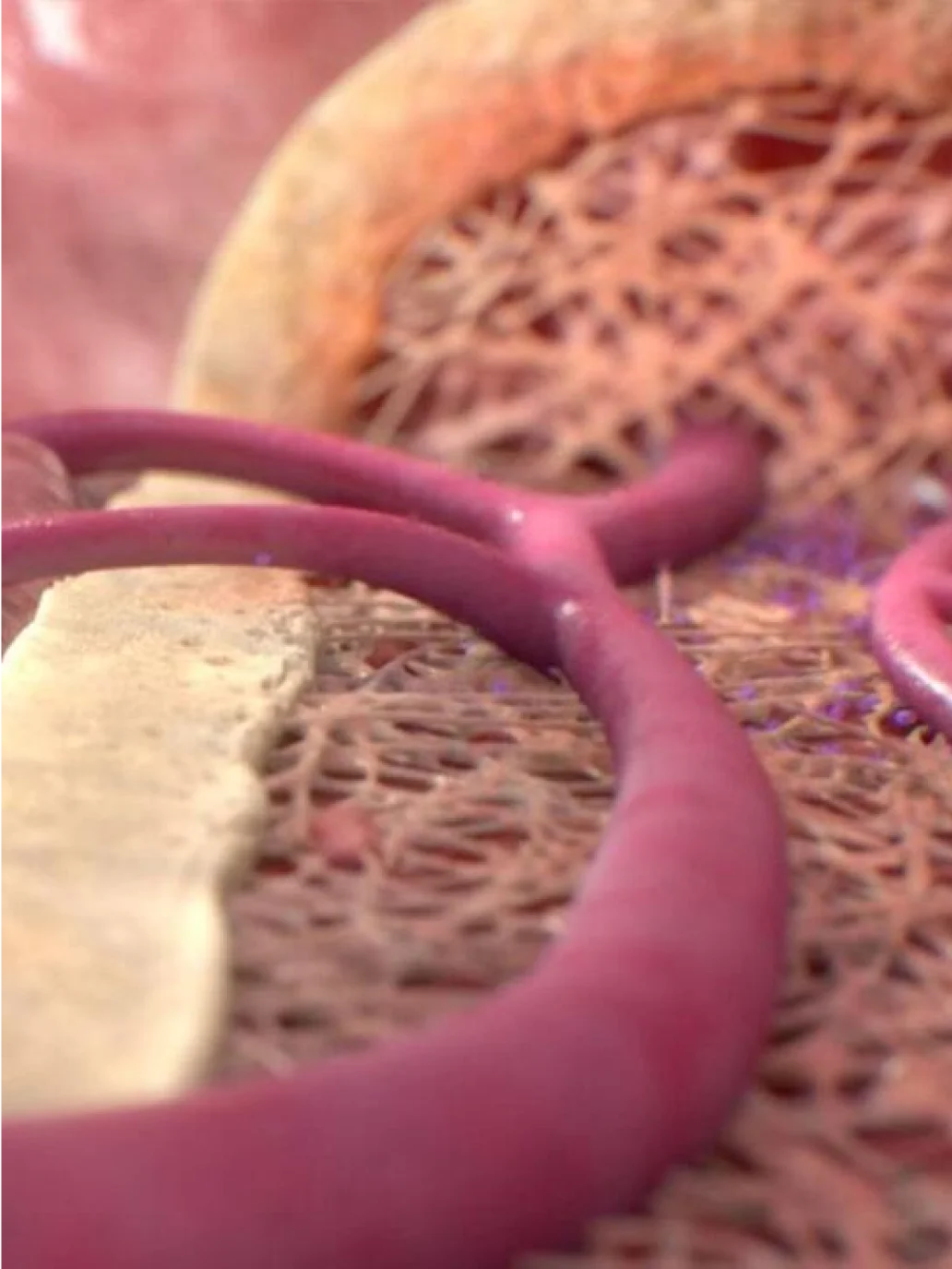
VYXEOS: Mechanism of action
Transcript
((Voice Over)) Acute myeloid leukemia, or AML, is a complex heterogeneous disease. Due to advanced age at the time of diagnosis and the need for aggressive treatment to achieve remission, AML is often associated with poor survival outcomes. Adults with t-AML and AML-MRC have a particularly poor prognosis.
The development of new treatment options and achieving meaningful improvements in treating these patients has been challenging. The 7+3 chemotherapy regimen has remained the foundation and has been the standard of care for AML induction for over 40 years.
As a pioneer in liposomal nanotechnology research, Dr. Lawrence Mayer has led the development of multiple liposomal products in the pharmaceutical industry. With VYXEOS, he saw an opportunity to answer the high unmet need for adult patients \with newly-diagnosed t-AML or AML-MRC.
((Dr. Mayer)) VYXEOS is the first FDA-approved dual-drug liposomal technology that encapsulates daunorubicin and cytarabine, the foundation drugs of the 7+3 regimen. VYXEOS was designed for simultaneous delivery of the 2 individual medications in 1 liposome at a fixed 1:5 molar ratio that is sustained in the bone marrow for a prolonged period of time.
How is VYXEOS different from the traditional 7+3 regimen? When administering daunorubicin and cytarabine individually, as with the 7+3 regimen, each drug has its own unique pharmacokinetics and distribution within the body. VYXEOS was developed to control the relative daunorubicin and cytarabine levels that reach leukemia cells.
((Voice Over)) Designed with dual-drug liposomal technology, VYXEOS is a nanoscale liposome of daunorubicin and cytarabine. Through extensive preclinical research, a molar ratio of one to five of daunorubicin to cytarabine was carefully selected, allowing for a synergistic effect at killing leukemia cells.
Pharmacokinetic studies have demonstrated that following a 90-minute intravenous infusion, VYXEOS exhibits a prolonged half-life with greater than 99% of daunorubicin and cytarabine in the plasma, remaining encapsulated within the liposomes.
Due to the pharmacokinetics and simultaneous delivery, with in vitro and in vivo studies, VYXEOS has been shown to deliver daunorubicin and cytarabine in a fixed one-to-five molar ratio to leukemia cells.
Based on animal models, the mechanism of action for VYXEOS is thought to occur in 3 main steps:
VYXEOS liposomes enter and persist in the bone marrow. The liposomes are taken up by leukemia cells to a greater extent than by normal bone marrow cells in a murine model. VYXEOS liposomes undergo degradation, releasing daunorubicin and cytarabine within the intracellular environment.
Once released within the intracellular environment, daunorubicin and cytarabine exert their antineoplastic activity. The 1:5 molar ratio of daunorubicin to cytarabine has been shown to have synergistic effects at killing leukemia cells in vitro and in animal models.
Dr. Arthur Louie is a board-certified clinical oncologist with over 30 years of experience in pharmaceutical research. He led the development of the clinical program for VYXEOS. He will now review some key efficacy and safety data for VYXEOS.
((Dr. Louie)) Based on experience throughout the clinical development program, we designed the pivotal phase 3 trial to look at VYXEOS in a population of adults with newly-diagnosed, therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC).
The pivotal phase 3 trial was a multicenter, open-label, active-controlled, superiority trial. In this trial, VYXEOS was studied in a head-to-head comparison versus the standard 7+3 (cytarabine and daunorubicin) regimen. The trial randomized 309 patients with newly-diagnosed t-AML or AML-MRC. This trial included patients with de novo AML with MDS-related cytogenetic abnormalities, AML with a history of myelodysplasia or chronic myelomonocytic leukemia, and t-AML.
The study established that VYXEOS is the first chemotherapy to demonstrate superior overall survival of 9.6 months vs 5.9 months for the control arm in adults with newly-diagnosed t-AML or AML-MRC.
((Voice Over)) Next, let’s review some safety data from the phase 3 trials. As shown in this table, reported adverse reactions were generally consistent with the known safety profile of daunorubicin and cytarabine therapy. Fatal treatment-emergent CNS hemorrhage not in the setting of progressive disease occurred in 2% of patients in the VYXEOS arm and 0.7% percent of patients in the control arm. VYXEOS was also associated with lower 30- and 60-day mortality rates compared to 7+3.
With its demonstrated improvement in overall survival compared to 7+3 in adult patients with newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes, VYXEOS represents a significant therapeutic advance for these AML patients.
((Voice Over)) Acute myeloid leukemia, or AML, is a complex heterogeneous disease. Due to advanced age at the time of diagnosis and the need for aggressive treatment to achieve remission, AML is often associated with poor survival outcomes. Adults with t-AML and AML-MRC have a particularly poor prognosis.
The development of new treatment options and achieving meaningful improvements in treating these patients has been challenging. The 7+3 chemotherapy regimen has remained the foundation and has been the standard of care for AML induction for over 40 years.
As a pioneer in liposomal nanotechnology research, Dr. Lawrence Mayer has led the development of multiple liposomal products in the pharmaceutical industry. With VYXEOS, he saw an opportunity to answer the high unmet need for adult patients \with newly-diagnosed t-AML or AML-MRC.
((Dr. Mayer)) VYXEOS is the first FDA-approved dual-drug liposomal technology that encapsulates daunorubicin and cytarabine, the foundation drugs of the 7+3 regimen. VYXEOS was designed for simultaneous delivery of the 2 individual medications in 1 liposome at a fixed 1:5 molar ratio that is sustained in the bone marrow for a prolonged period of time.
How is VYXEOS different from the traditional 7+3 regimen? When administering daunorubicin and cytarabine individually, as with the 7+3 regimen, each drug has its own unique pharmacokinetics and distribution within the body. VYXEOS was developed to control the relative daunorubicin and cytarabine levels that reach leukemia cells.
((Voice Over)) Designed with dual-drug liposomal technology, VYXEOS is a nanoscale liposome of daunorubicin and cytarabine. Through extensive preclinical research, a molar ratio of one to five of daunorubicin to cytarabine was carefully selected, allowing for a synergistic effect at killing leukemia cells.
Pharmacokinetic studies have demonstrated that following a 90-minute intravenous infusion, VYXEOS exhibits a prolonged half-life with greater than 99% of daunorubicin and cytarabine in the plasma, remaining encapsulated within the liposomes.
Due to the pharmacokinetics and simultaneous delivery, with in vitro and in vivo studies, VYXEOS has been shown to deliver daunorubicin and cytarabine in a fixed one-to-five molar ratio to leukemia cells.
Based on animal models, the mechanism of action for VYXEOS is thought to occur in 3 main steps:
VYXEOS liposomes enter and persist in the bone marrow. The liposomes are taken up by leukemia cells to a greater extent than by normal bone marrow cells in a murine model. VYXEOS liposomes undergo degradation, releasing daunorubicin and cytarabine within the intracellular environment.
Once released within the intracellular environment, daunorubicin and cytarabine exert their antineoplastic activity. The 1:5 molar ratio of daunorubicin to cytarabine has been shown to have synergistic effects at killing leukemia cells in vitro and in animal models.
Dr. Arthur Louie is a board-certified clinical oncologist with over 30 years of experience in pharmaceutical research. He led the development of the clinical program for VYXEOS. He will now review some key efficacy and safety data for VYXEOS.
((Dr. Louie)) Based on experience throughout the clinical development program, we designed the pivotal phase 3 trial to look at VYXEOS in a population of adults with newly-diagnosed, therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC).
The pivotal phase 3 trial was a multicenter, open-label, active-controlled, superiority trial. In this trial, VYXEOS was studied in a head-to-head comparison versus the standard 7+3 (cytarabine and daunorubicin) regimen. The trial randomized 309 patients with newly-diagnosed t-AML or AML-MRC. This trial included patients with de novo AML with MDS-related cytogenetic abnormalities, AML with a history of myelodysplasia or chronic myelomonocytic leukemia, and t-AML.
The study established that VYXEOS is the first chemotherapy to demonstrate superior overall survival of 9.6 months vs 5.9 months for the control arm in adults with newly-diagnosed t-AML or AML-MRC.
((Voice Over)) Next, let’s review some safety data from the phase 3 trials. As shown in this table, reported adverse reactions were generally consistent with the known safety profile of daunorubicin and cytarabine therapy. Fatal treatment-emergent CNS hemorrhage not in the setting of progressive disease occurred in 2% of patients in the VYXEOS arm and 0.7% percent of patients in the control arm. VYXEOS was also associated with lower 30- and 60-day mortality rates compared to 7+3.
With its demonstrated improvement in overall survival compared to 7+3 in adult patients with newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes, VYXEOS represents a significant therapeutic advance for these AML patients.
10:14 MINS

Speaker: Jonathan A. Abbas, MD
VYXEOS Phase 3 clinical study review
Transcript
((Dr. Abbas))
Hello, I’m Dr Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
CPX-351 (or VYXEOS) is a liposomal combination of a synergistic ratio of daunorubicin and cytarabine and is the only treatment recommended in the NCCN Guidelines® for induction in patients ≥60 years of age with t-AML or antecedent MDS/CMML or AML-MRC being a category 1 recommendation.
The 1:5 molar ratio of daunorubicin to cytarabine has been shown to have synergistic effects at killing leukemia cells in vitro and in murine models.
VYXEOS was approved by the FDA based on the results of a large Phase 3 trial in newly-diagnosed patients with t-AML or AML-MRC.
Patients could receive up to 2 cycles of induction and 2 cycles of consolidation in each arm. A second induction was highly recommended for patients who did not achieve a response and was mandatory for patients achieving >50% reduction in percent blasts.
VYXEOS is the first chemotherapy to demonstrate superior overall survival versus 7+3 in adults with newly-diagnosed t-AML or AML-MRC.
The Phase 3 multicenter, open-label, randomized study of VYXEOS versus 7+3 included 309 patients with newly-diagnosed t-AML or AML-MRC, aged 60 to 75 years.
Primary endpoint was OS.
Patient and disease characteristics were similar across both the VYXEOS and 7+3 treatment study arms.
Overall survival is more than double at 5 years with VYXEOS at 18% versus 8% with 7+3, based on KM estimate.
The reported adverse reactions for VYXEOS in the Phase 3 trial were generally consistent with the known safety profile of cytarabine and daunorubicin therapy.
VYXEOS was associated with lower 30- and 60-day mortality rates compared to treatment with 7+3.
6% of patients in both the VYXEOS arm and the control arm had a fatal adverse reaction on treatment or within 30 days of therapy that was not in the setting of progressive disease. Overall, all-cause 30-day mortality was 6% in the VYXEOS arm and 11% in the control arm During the first 60 days of the study, 14% of patients in the VYXEOS arm and 21% of patients in the 7+3 arm died Fatal adverse reactions in the VYXEOS arm included infection, CNS hemorrhage, and respiratory failure
For years we have known that sAML is one of the most challenging types of AML to treat because so few of the diseases actually go into a remission.
Historically we’ve always used 7+3 as our induction due to the lack of superiority of any regimen in a randomized clinical trial, and this is why the study with VYXEOS is so significant because now we see not only improved overall survival but we also see a higher percentage of patients achieving that first remission, which opens the door for the potential curative stem cell transplant.
Hello, I’m Dr Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
CPX-351 (or VYXEOS) is a liposomal combination of a synergistic ratio of daunorubicin and cytarabine and is the only treatment recommended in the NCCN Guidelines® for induction in patients ≥60 years of age with t-AML or antecedent MDS/CMML or AML-MRC being a category 1 recommendation.
The 1:5 molar ratio of daunorubicin to cytarabine has been shown to have synergistic effects at killing leukemia cells in vitro and in murine models.
VYXEOS was approved by the FDA based on the results of a large Phase 3 trial in newly-diagnosed patients with t-AML or AML-MRC.
Patients could receive up to 2 cycles of induction and 2 cycles of consolidation in each arm. A second induction was highly recommended for patients who did not achieve a response and was mandatory for patients achieving >50% reduction in percent blasts.
VYXEOS is the first chemotherapy to demonstrate superior overall survival versus 7+3 in adults with newly-diagnosed t-AML or AML-MRC.
The Phase 3 multicenter, open-label, randomized study of VYXEOS versus 7+3 included 309 patients with newly-diagnosed t-AML or AML-MRC, aged 60 to 75 years.
Primary endpoint was OS.
Patient and disease characteristics were similar across both the VYXEOS and 7+3 treatment study arms.
Overall survival is more than double at 5 years with VYXEOS at 18% versus 8% with 7+3, based on KM estimate.
The reported adverse reactions for VYXEOS in the Phase 3 trial were generally consistent with the known safety profile of cytarabine and daunorubicin therapy.
VYXEOS was associated with lower 30- and 60-day mortality rates compared to treatment with 7+3.
6% of patients in both the VYXEOS arm and the control arm had a fatal adverse reaction on treatment or within 30 days of therapy that was not in the setting of progressive disease. Overall, all-cause 30-day mortality was 6% in the VYXEOS arm and 11% in the control arm During the first 60 days of the study, 14% of patients in the VYXEOS arm and 21% of patients in the 7+3 arm died Fatal adverse reactions in the VYXEOS arm included infection, CNS hemorrhage, and respiratory failure
For years we have known that sAML is one of the most challenging types of AML to treat because so few of the diseases actually go into a remission.
Historically we’ve always used 7+3 as our induction due to the lack of superiority of any regimen in a randomized clinical trial, and this is why the study with VYXEOS is so significant because now we see not only improved overall survival but we also see a higher percentage of patients achieving that first remission, which opens the door for the potential curative stem cell transplant.


preparation, dosing, & administration
9:49 MINS
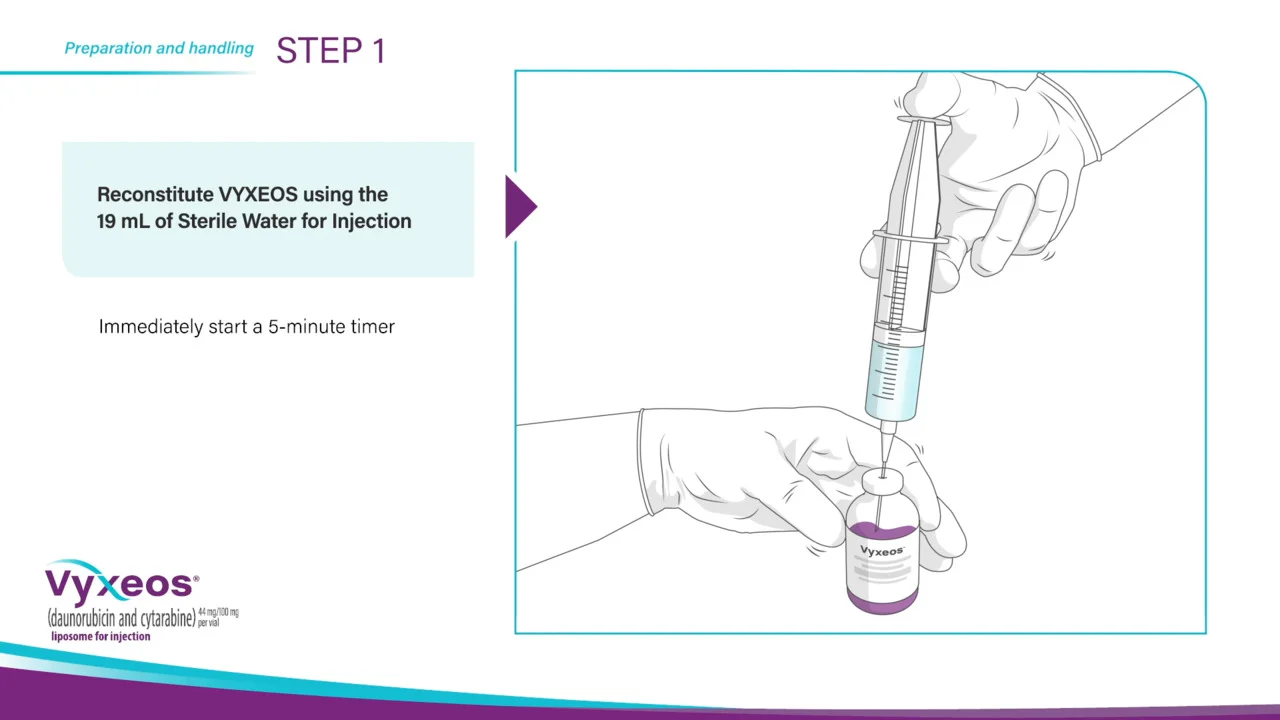
VYXEOS: Preparation & administration
Transcript
((Voice Over)) This video provides a step-by-step guide on how to prepare and administer VYXEOS.
VYXEOS requires reconstitution and further dilution prior to intravenous infusion. For the reconstitution of one VYXEOS vial, you will need a sterile syringe, needle, Sterile Water for Injection, VYXEOS, and an IV bag containing 500 milliliters of either 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP. VYXEOS vials should be refrigerated until needed for preparation. VYXEOS is a hazardous drug. Follow applicable special handling and disposal procedures.
VYXEOS contains both daunorubicin and cytarabine at a fixed molar ratio. The calculation to determine the volume of VYXEOS required per dose is based on the dose of daunorubicin. You will then use the patient’s body surface area, or BSA, to complete the calculation. To determine the volume of VYXEOS needed for the dose, multiply the patient’s BSA by the daunorubicin dose, then divide by 2.2 milligrams per milliliter. After reconstitution and prior to dilution, each vial of VYXEOS will contain 20 milliliters for administration. Each milliliter will contain 2.2 milligrams of daunorubicin and 5 milligrams of cytarabine.
Based on the volume required to prepare the dose, remove the appropriate number of VYXEOS vials from the refrigerator.
Let the vials rest for 30 minutes, allowing them to equilibrate to room temperature.
VYXEOS is supplied as a sterile, preservative-free, purple lyophilized cake.
Now that you have completed the setup, you can begin preparing VYXEOS for administration. Once the VYXEOS vials have reached room temperature, withdraw 19 milliliters of Sterile Water for Injection, using aseptic procedures.
Reconstitute VYXEOS with the 19 milliliters of Sterile Water for Injection and immediately start a 5-minute timer.
Begin gently swirling the contents of the vial for 5 minutes, while inverting every 30 seconds. Be sure to continue swirling the vial while it is inverted.
It is very important not to heat, vortex, or shake the vial at any time during this process.
After reconstitution, let the vials rest for 15 minutes.
The reconstituted product should be a purple, opaque, homogeneous dispersion, essentially free from particulates. Use the reconsistuted solution immediately. If needed, store the reconstituted product in the vial refrigerated at 2 degrees Celsius to 8 degrees Celsius, or 36 degrees Fahrenheit to 46 degrees Fahrenheit, for up to 4 hours. Note that the reconstituted product in the vial and the reconstituted product which has been diluted into an infusion solution are stable for a total of 4 hours (not 4 hours each) when stored at 2°C to 8°C.
After the 15-minute rest period, gently invert each vial 5 times for further dilution, then aseptically withdraw the calculated volume of reconstituted product from the vials with a sterile syringe.
Transfer the calculated volume into an infusion bag containing 500 milliliters of 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP. Discard any unused portion or residual product remaining in the vial, and do not save any unused portions for later administration.
Gently invert the IV bag to mix the solution.
This process should result in a deep purple, translucent solution. Prior to administration, inspect the solution in the IV bag to ensure that it is a homogeneous dispersion, free from any visible particulates. Only solutions without visible particles should be used. If the diluted infusion solution is not used immediately, store in the refrigerator at 2 degrees Celsius to 8 degrees Celsius, or 36 degrees Fahrenheit to 46 degrees Fahrenheit, for up to 4 hours. If the reconstituted solution in the vial was stored for 4 hours, the diluted infusion solution must be used immediately and cannot be stored for an additional 4 hours.
Administer VYXEOS by constant IV infusion over 90 minutes via an infusion pump through a central venous catheter or a peripherally inserted central catheter. Do not mix VYXEOS with or administer it as an infusion with other drugs.
After administration, flush the line with 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP.
((Voice Over)) This video provides a step-by-step guide on how to prepare and administer VYXEOS.
VYXEOS requires reconstitution and further dilution prior to intravenous infusion. For the reconstitution of one VYXEOS vial, you will need a sterile syringe, needle, Sterile Water for Injection, VYXEOS, and an IV bag containing 500 milliliters of either 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP. VYXEOS vials should be refrigerated until needed for preparation. VYXEOS is a hazardous drug. Follow applicable special handling and disposal procedures.
VYXEOS contains both daunorubicin and cytarabine at a fixed molar ratio. The calculation to determine the volume of VYXEOS required per dose is based on the dose of daunorubicin. You will then use the patient’s body surface area, or BSA, to complete the calculation. To determine the volume of VYXEOS needed for the dose, multiply the patient’s BSA by the daunorubicin dose, then divide by 2.2 milligrams per milliliter. After reconstitution and prior to dilution, each vial of VYXEOS will contain 20 milliliters for administration. Each milliliter will contain 2.2 milligrams of daunorubicin and 5 milligrams of cytarabine.
Based on the volume required to prepare the dose, remove the appropriate number of VYXEOS vials from the refrigerator.
Let the vials rest for 30 minutes, allowing them to equilibrate to room temperature.
VYXEOS is supplied as a sterile, preservative-free, purple lyophilized cake.
Now that you have completed the setup, you can begin preparing VYXEOS for administration. Once the VYXEOS vials have reached room temperature, withdraw 19 milliliters of Sterile Water for Injection, using aseptic procedures.
Reconstitute VYXEOS with the 19 milliliters of Sterile Water for Injection and immediately start a 5-minute timer.
Begin gently swirling the contents of the vial for 5 minutes, while inverting every 30 seconds. Be sure to continue swirling the vial while it is inverted.
It is very important not to heat, vortex, or shake the vial at any time during this process.
After reconstitution, let the vials rest for 15 minutes.
The reconstituted product should be a purple, opaque, homogeneous dispersion, essentially free from particulates. Use the reconsistuted solution immediately. If needed, store the reconstituted product in the vial refrigerated at 2 degrees Celsius to 8 degrees Celsius, or 36 degrees Fahrenheit to 46 degrees Fahrenheit, for up to 4 hours. Note that the reconstituted product in the vial and the reconstituted product which has been diluted into an infusion solution are stable for a total of 4 hours (not 4 hours each) when stored at 2°C to 8°C.
After the 15-minute rest period, gently invert each vial 5 times for further dilution, then aseptically withdraw the calculated volume of reconstituted product from the vials with a sterile syringe.
Transfer the calculated volume into an infusion bag containing 500 milliliters of 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP. Discard any unused portion or residual product remaining in the vial, and do not save any unused portions for later administration.
Gently invert the IV bag to mix the solution.
This process should result in a deep purple, translucent solution. Prior to administration, inspect the solution in the IV bag to ensure that it is a homogeneous dispersion, free from any visible particulates. Only solutions without visible particles should be used. If the diluted infusion solution is not used immediately, store in the refrigerator at 2 degrees Celsius to 8 degrees Celsius, or 36 degrees Fahrenheit to 46 degrees Fahrenheit, for up to 4 hours. If the reconstituted solution in the vial was stored for 4 hours, the diluted infusion solution must be used immediately and cannot be stored for an additional 4 hours.
Administer VYXEOS by constant IV infusion over 90 minutes via an infusion pump through a central venous catheter or a peripherally inserted central catheter. Do not mix VYXEOS with or administer it as an infusion with other drugs.
After administration, flush the line with 0.9 percent Sodium Chloride Injection, USP or 5 percent Dextrose Injection, USP.
7:30 MINS

Speaker: Jonathan A. Abbas, MD
VYXEOS: Dosing and administration
(a physician’s perspective)
(a physician’s perspective)
Transcript
((Dr. Abbas)) Hello, I’m Dr Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
Prior to initiating each cycle of VYXEOS, calculate the prior cumulative anthracycline exposure for the patient. Administer prophylactic anti-emetics before treatment with VYXEOS.
Prior to initiating induction, assess cardiac function and obtain liver and renal function studies.
After induction therapy with VYXEOS, bone marrow assessment should be conducted to determine if the patient has achieved a CR.
For patients who do not achieve remission with the first induction cycle, a second induction cycle may be administered 2 to 5 weeks after the first if there was no unacceptable toxicity with VYXEOS.
Administer the first consolidation cycle 5 to 8 weeks after the start of the last induction.
Administer the second consolidation cycle 5 to 8 weeks after the start of the first consolidation cycle in patients who do not show disease progression or unacceptable toxicity to VYXEOS.
((Dr. Abbas)) Hello, I’m Dr Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
Prior to initiating each cycle of VYXEOS, calculate the prior cumulative anthracycline exposure for the patient. Administer prophylactic anti-emetics before treatment with VYXEOS.
Prior to initiating induction, assess cardiac function and obtain liver and renal function studies.
After induction therapy with VYXEOS, bone marrow assessment should be conducted to determine if the patient has achieved a CR.
For patients who do not achieve remission with the first induction cycle, a second induction cycle may be administered 2 to 5 weeks after the first if there was no unacceptable toxicity with VYXEOS.
Administer the first consolidation cycle 5 to 8 weeks after the start of the last induction.
Administer the second consolidation cycle 5 to 8 weeks after the start of the first consolidation cycle in patients who do not show disease progression or unacceptable toxicity to VYXEOS.


Outpatient Administration
4:59 MINS

Speaker: Rami Komrokji, MD
Inpatient/outpatient (IPOP) treatment plan
Transcript
((Rami Komrokji, MD)) Recently, the landscape of acute myeloid leukemia treatment has been changing.
More institutions are moving to an outpatient treatment approach and, in my experience, a part of this consideration is due to volume in hospitals and patient preference.
Treatment in the inpatient/outpatient setting, also known as an IPOP treatment plan, enables appropriate patients to receive induction in an outpatient setting, with a planned inpatient admission for continued monitoring and subsequent management of complications that may arise.
Furthermore, the use of oral prophylactic antimicrobial agents has increased over the last several years, and outpatient transfusion support has become standard.
Due to these advancements, some patients with AML may now be supported in an IPOP setting.
The potential benefit of an IPOP plan is that patients can be hospitalized for less time during treatment.
((Rami Komrokji, MD)) Recently, the landscape of acute myeloid leukemia treatment has been changing.
More institutions are moving to an outpatient treatment approach and, in my experience, a part of this consideration is due to volume in hospitals and patient preference.
Treatment in the inpatient/outpatient setting, also known as an IPOP treatment plan, enables appropriate patients to receive induction in an outpatient setting, with a planned inpatient admission for continued monitoring and subsequent management of complications that may arise.
Furthermore, the use of oral prophylactic antimicrobial agents has increased over the last several years, and outpatient transfusion support has become standard.
Due to these advancements, some patients with AML may now be supported in an IPOP setting.
The potential benefit of an IPOP plan is that patients can be hospitalized for less time during treatment.
4:54 MINS

Speaker: Sara M. Tinsley, PhD, ARNP, AOCN
Inpatient/outpatient (IPOP) infrastructure
Transcript
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) In an IPOP treatment approach, appropriate patients can receive induction in an outpatient setting, followed by a planned admission.
The patient is seen daily for monitoring.
There are a number of team members needed to support an IPOP approach.
A nurse practitioner or physician assistant should be available to see the patient daily.
An attending physician should also be available for any consultations.
A social worker may also play a role in IPOP coordination.
They can help assess the suitability of the patient's transportation, local support system, and anything else that may impact the patient’s participation in IPOP treatment.
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) In an IPOP treatment approach, appropriate patients can receive induction in an outpatient setting, followed by a planned admission.
The patient is seen daily for monitoring.
There are a number of team members needed to support an IPOP approach.
A nurse practitioner or physician assistant should be available to see the patient daily.
An attending physician should also be available for any consultations.
A social worker may also play a role in IPOP coordination.
They can help assess the suitability of the patient's transportation, local support system, and anything else that may impact the patient’s participation in IPOP treatment.
5:23 MINS

Speaker: Rami Komrokji, MD
Inpatient/outpatient (IPOP) considerations
Transcript
((Rami Komrokji, MD)) Some larger institutions are conducting IPOP administration more frequently, but there are some elements to consider before proceeding with this treatment plan, such as patient- and disease-related factors and the institution’s infrastructure.
It is important to assess whether the patient's disease is in proliferative status or they have neutropenia or any other major comorbidities, as these factors would likely remove them as candidates for IPOP administration.
In addition, for our institution, patients with renal failure are typically excluded from IPOP treatment with VYXEOS.
Another consideration for IPOP is that the patient should be able to stay local for induction on Days 1, 3, and 5 of treatment prior to planned admission.
The institution must also have the necessary infrastructure to support an IPOP approach, which may include daily monitoring and transfusion support, with blood transfusions given as needed.
Daily monitoring may include clinical evaluation and blood tests, such as complete blood count, comprehensive metabolic panel, and uric acid and phosphorus levels.
Typically, patients will spend a half day in the infusion center and be given fluids for hydration and other supportive treatments as needed.
((Rami Komrokji, MD)) Some larger institutions are conducting IPOP administration more frequently, but there are some elements to consider before proceeding with this treatment plan, such as patient- and disease-related factors and the institution’s infrastructure.
It is important to assess whether the patient's disease is in proliferative status or they have neutropenia or any other major comorbidities, as these factors would likely remove them as candidates for IPOP administration.
In addition, for our institution, patients with renal failure are typically excluded from IPOP treatment with VYXEOS.
Another consideration for IPOP is that the patient should be able to stay local for induction on Days 1, 3, and 5 of treatment prior to planned admission.
The institution must also have the necessary infrastructure to support an IPOP approach, which may include daily monitoring and transfusion support, with blood transfusions given as needed.
Daily monitoring may include clinical evaluation and blood tests, such as complete blood count, comprehensive metabolic panel, and uric acid and phosphorus levels.
Typically, patients will spend a half day in the infusion center and be given fluids for hydration and other supportive treatments as needed.


supportive care and aml management
4:50 MINS

Speaker: Sara M. Tinsley, PhD, ARNP, AOCN
Assisting count recovery
Transcript
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) Because neutrophils and platelets may take longer to recover after treatment with VYXEOS, granulocyte-colony stimulating factor and platelet transfusions may be given to assist recovery.
For a patient whose counts have not recovered, we see them twice a week until fully recovered.
During their visits, we look for bleeding and signs of infection and we administer blood transfusions as required.
Generally, we look for patients to have fewer than 5% blasts before granulocyte-colony stimulating factor is given to aid with recovery, but this may vary by institution.
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) Because neutrophils and platelets may take longer to recover after treatment with VYXEOS, granulocyte-colony stimulating factor and platelet transfusions may be given to assist recovery.
For a patient whose counts have not recovered, we see them twice a week until fully recovered.
During their visits, we look for bleeding and signs of infection and we administer blood transfusions as required.
Generally, we look for patients to have fewer than 5% blasts before granulocyte-colony stimulating factor is given to aid with recovery, but this may vary by institution.
4:50 MINS

Speaker: Sara M. Tinsley, PhD, ARNP, AOCN
Red blood cell transfusions
Transcript
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) The need for red blood cell transfusions takes into consideration a number of patient specific factors.
We monitor the patient's underlying condition to determine the appropriate threshold at which to start red blood cell transfusions.
Some patients may have higher thresholds for initiating transfusions, for example, if they have significant cardio or pulmonary disease.
At our institution, we generally say the lower limit for hemoglobin is 7 g/dL to require a standing order for transfusion, but this will vary by each institution and their specific protocol.
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) The need for red blood cell transfusions takes into consideration a number of patient specific factors.
We monitor the patient's underlying condition to determine the appropriate threshold at which to start red blood cell transfusions.
Some patients may have higher thresholds for initiating transfusions, for example, if they have significant cardio or pulmonary disease.
At our institution, we generally say the lower limit for hemoglobin is 7 g/dL to require a standing order for transfusion, but this will vary by each institution and their specific protocol.
5:00 MINS

Speaker: Sara M. Tinsley, Ph.D, ARNP, AOCN
Bone marrow assessments
Transcript
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) VYXEOS has demonstrated in animal models that it is delivered to the bone marrow, where it is taken up by leukemia cells to a greater extent than by normal cells and exerts an antileukemic effect.
The extended half-life of VYXEOS allows for greater drug exposure within the plasma and bone marrow.
At our institution, we assess bone marrow between Days 14 and 21, and we usually wait until Day 21 to allow the drug to work as long as possible.
Since we don’t want to extract bone marrow too often because it can be taxing on the patient, we may consider assessing the marrow based on the attending physician’s schedule—aligning it with the physician who has been closest to the patient's progress.
((Sara M. Tinsley, Ph.D., ARNP, AOCN)) VYXEOS has demonstrated in animal models that it is delivered to the bone marrow, where it is taken up by leukemia cells to a greater extent than by normal cells and exerts an antileukemic effect.
The extended half-life of VYXEOS allows for greater drug exposure within the plasma and bone marrow.
At our institution, we assess bone marrow between Days 14 and 21, and we usually wait until Day 21 to allow the drug to work as long as possible.
Since we don’t want to extract bone marrow too often because it can be taxing on the patient, we may consider assessing the marrow based on the attending physician’s schedule—aligning it with the physician who has been closest to the patient's progress.


remission and transplant
4:45 MINS

Speaker: Rami Komrokji, MD
Complete remission
Transcript
((Rami Komrokji, MD)) Complete remission, or CR, refers to the reduction of myloblasts to under 5% with complete count recovery.
CR correlates with better outcomes. When assessing patients for transplant, doctors are looking for patients in CR rather than those with active disease.
In my experience, I administer consolidation with VYXEOS prior to hematopoietic stem cell transplant to help maintain remission while aspects of transplant, such as finding an available donor, are finalized.
With VYXEOS, up to 2 cycles of consolidation can be administered.
((Rami Komrokji, MD)) Complete remission, or CR, refers to the reduction of myloblasts to under 5% with complete count recovery.
CR correlates with better outcomes. When assessing patients for transplant, doctors are looking for patients in CR rather than those with active disease.
In my experience, I administer consolidation with VYXEOS prior to hematopoietic stem cell transplant to help maintain remission while aspects of transplant, such as finding an available donor, are finalized.
With VYXEOS, up to 2 cycles of consolidation can be administered.
8:45 MINS

Speaker: Jonathan A. Abbas, MD
Hematopoietic stem cell transplant
(a physician's perspective)
(a physician's perspective)
Transcript
((Dr. Abbas)) Hello, I’m Dr. Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
Due to poor prognosis in sAML patients, the goal should be to get appropriate patients to HSCT because:
Patients with AML-MRC and t-AML who undergo HSCT after chemotherapy have been shown to have better outcomes compared with chemotherapy alone The outcomes of older AML patients undergoing hematopoietic stem cell transplant has improved An increasing amount of older patients may be eligible for stem cell transplant One of the major breakthroughs in hematopoietic stem cell transplant in the last 20 years is the use of reduced intensity conditioning to be able to transplant patients in their 60s and 70s. As this is the age range in which most sAML patients present, this means that now these patients have a potential pathway to a cure. Of course, we cannot perform a stem cell transplant on a AML patient unless they have achieved a complete remission. By using VYXEOS, we now open the door to a potential curative transplant for patients with sAML in their 60s and 70s.
Factors to consider for hematopoietic stem cell transplant eligibility include: comorbidities, older age, disease biology as in refractory or a short period of remission, physical impairment or poor performance status.
In the Phase 3 trial, 35% of VYXEOS-treated patients received a hematopoietic stem cell transplant after treatment compared to 25% of patients treated with 7+3.
Furthermore, a greater proportion of patients who achieved first CR with VYXEOS (20%) vs 7+3 (12%) subsequently underwent hematopoietic stem cell transplant.
Managing patients with secondary AML can be challenging, but I hope what I discussed today can offer clinicians in the community setting additional treatment options that can help improve outcomes for their secondary AML patients. Thank you.
((Dr. Abbas)) Hello, I’m Dr. Jonathan Abbas. I’m the director of the acute leukemia and blood cancer program with Tennessee Oncology in Nashville, Tennessee. I specialize in hematologic malignancies, specifically acute leukemia, and my center treats over a hundred patients a year with aggressive chemotherapy for AML many of whom have sAML, including t-AML and AML-MRC.
Due to poor prognosis in sAML patients, the goal should be to get appropriate patients to HSCT because:
Patients with AML-MRC and t-AML who undergo HSCT after chemotherapy have been shown to have better outcomes compared with chemotherapy alone The outcomes of older AML patients undergoing hematopoietic stem cell transplant has improved An increasing amount of older patients may be eligible for stem cell transplant One of the major breakthroughs in hematopoietic stem cell transplant in the last 20 years is the use of reduced intensity conditioning to be able to transplant patients in their 60s and 70s. As this is the age range in which most sAML patients present, this means that now these patients have a potential pathway to a cure. Of course, we cannot perform a stem cell transplant on a AML patient unless they have achieved a complete remission. By using VYXEOS, we now open the door to a potential curative transplant for patients with sAML in their 60s and 70s.
Factors to consider for hematopoietic stem cell transplant eligibility include: comorbidities, older age, disease biology as in refractory or a short period of remission, physical impairment or poor performance status.
In the Phase 3 trial, 35% of VYXEOS-treated patients received a hematopoietic stem cell transplant after treatment compared to 25% of patients treated with 7+3.
Furthermore, a greater proportion of patients who achieved first CR with VYXEOS (20%) vs 7+3 (12%) subsequently underwent hematopoietic stem cell transplant.
Managing patients with secondary AML can be challenging, but I hope what I discussed today can offer clinicians in the community setting additional treatment options that can help improve outcomes for their secondary AML patients. Thank you.


Patient stories
7:21 MINS

Tim, patient with AML-MRC
Transcript of Tim’s story
TIM: When I was diagnosed I was absolutely shocked. I went in for a routine physical. The doctor did some blood work for me and called me up about a week later and said there must be something wrong in the lab you've gotta come back in and do a retest.
Kathy: I had been noticing some changes in him. You know you don’t know whether it’s because we were aging or what we were doing, so then to hear the diagnosis it was like OK how did we miss, like did I miss something, was there a way I could have done something better.
Caitlin: I don’t think anything can prepare you for your Dad sitting you down and telling you that he’s sick. From the minute he told us, it was just like taking information in with a firehose….
TIM: I initially was diagnosed with MDS. I had a clinical trial that unfortunately didn’t work. My MDS was moved to AML and that’s when my doctor prescribed or suggested I move to Vyxeos.
Kathy: Tim really decided from the moment he was diagnosed that he was going to beat this…. And he wasn’t going to become the patient. He was going to take all the responsibility for what he needed to to get through it.
Caitlin: We all heard what he said and we all knew that he was going to go at this with everything he had and that I think we all decided that we were going to do everything we could to support him.
TIM: My approach to how I was going to battle the illness was to to fight it to, to work really hard and do everything I could to put myself in the best position to be successful.
TIM: It was important to me to have a routine. I walked three times a day...they had a white board in my hospital room that every morning they would come in and give me the the white count, the red count. Monitoring that everyday and watching it kept me motivated and helped me stay focused on getting well.
TIM: I was fortunate enough to only need one cycle of Vyxeos which led to remission.
TIM: The doctor said that I would have to have a transplant in order to survive my leukemia so that was the only option that I had, which motivated me even more to do my part to get to transplant.
TIM: I was very very lucky to have a number of exact matches. We set a date for the end of the year, in December, and worked towards that goal.
TIM: I think my advice for for people is to prepare yourself for this journey. It is difficult but it’s not impossible. I would tell everyone to try to get in the best shape they could in the time they have in preparing.
TIM: I would definitely set up a group that will support you - friends, family. I started a blog which was very important to me. It helped me communicate my status to important people in my life and it also gave them the opportunity to communicate with me, encouraging me so that was very important…. Having a diary or a journal is important because it keeps you true to yourself… it helps you keep fighting.
TIM: Life is definitely different than it was before I was diagnosed. And being in full remission I think I have a different perspective in life. I think every day is a gift. Enjoying people and and everyday —making the best out of everyday is very important to me now.
TIM: When I was diagnosed I was absolutely shocked. I went in for a routine physical. The doctor did some blood work for me and called me up about a week later and said there must be something wrong in the lab you've gotta come back in and do a retest.
Kathy: I had been noticing some changes in him. You know you don’t know whether it’s because we were aging or what we were doing, so then to hear the diagnosis it was like OK how did we miss, like did I miss something, was there a way I could have done something better.
Caitlin: I don’t think anything can prepare you for your Dad sitting you down and telling you that he’s sick. From the minute he told us, it was just like taking information in with a firehose….
TIM: I initially was diagnosed with MDS. I had a clinical trial that unfortunately didn’t work. My MDS was moved to AML and that’s when my doctor prescribed or suggested I move to Vyxeos.
Kathy: Tim really decided from the moment he was diagnosed that he was going to beat this…. And he wasn’t going to become the patient. He was going to take all the responsibility for what he needed to to get through it.
Caitlin: We all heard what he said and we all knew that he was going to go at this with everything he had and that I think we all decided that we were going to do everything we could to support him.
TIM: My approach to how I was going to battle the illness was to to fight it to, to work really hard and do everything I could to put myself in the best position to be successful.
TIM: It was important to me to have a routine. I walked three times a day...they had a white board in my hospital room that every morning they would come in and give me the the white count, the red count. Monitoring that everyday and watching it kept me motivated and helped me stay focused on getting well.
TIM: I was fortunate enough to only need one cycle of Vyxeos which led to remission.
TIM: The doctor said that I would have to have a transplant in order to survive my leukemia so that was the only option that I had, which motivated me even more to do my part to get to transplant.
TIM: I was very very lucky to have a number of exact matches. We set a date for the end of the year, in December, and worked towards that goal.
TIM: I think my advice for for people is to prepare yourself for this journey. It is difficult but it’s not impossible. I would tell everyone to try to get in the best shape they could in the time they have in preparing.
TIM: I would definitely set up a group that will support you - friends, family. I started a blog which was very important to me. It helped me communicate my status to important people in my life and it also gave them the opportunity to communicate with me, encouraging me so that was very important…. Having a diary or a journal is important because it keeps you true to yourself… it helps you keep fighting.
TIM: Life is definitely different than it was before I was diagnosed. And being in full remission I think I have a different perspective in life. I think every day is a gift. Enjoying people and and everyday —making the best out of everyday is very important to me now.
5:36 MINS

John, patient with AML-MRC
Transcript of John’s story
John: When I got diagnosed with AML ah nobody in my family had it.
John: I was told it was pretty rare and fast-moving.
Cherie: It was daunting, it was enriching.
Cherie: We learned a lot, and in many ways we became closer.
John: Going in there I knew I was on a clinical trial.
Cherie: We were just so blessed to be there at the right time at the right place and have a spot that they wanted an old man to try.
John: Being on a clinical trial was… well it saved me, really.
John: Being in the hospital you know you’re going to have time other than being interrupted every four hours for a lot of things and I decided I’m going to keep a log of what happens. You-you know you’re going to have time in between and so I did a lot of walking and they told me when I checked into that hospital that 11 times around the out- the per-perimeter was a mile. So I set goals, I’m gonna do my exercise.
John: There were a few days where I had trouble getting up and ah I did walk around but I didn’t record… I mentioned that I didn’t do any laps. What I call laps are when I was really moving… and ah I did get up and walk around very slowly and… those days I didn’t do much. But I had to try and do something.
John: My advice for somebody going through this is have the support, have a positive positive attitude, keep in shape, the best shape you can be.
John: Ah We - we look at it like it’s a new day every day.
John: When I got diagnosed with AML ah nobody in my family had it.
John: I was told it was pretty rare and fast-moving.
Cherie: It was daunting, it was enriching.
Cherie: We learned a lot, and in many ways we became closer.
John: Going in there I knew I was on a clinical trial.
Cherie: We were just so blessed to be there at the right time at the right place and have a spot that they wanted an old man to try.
John: Being on a clinical trial was… well it saved me, really.
John: Being in the hospital you know you’re going to have time other than being interrupted every four hours for a lot of things and I decided I’m going to keep a log of what happens. You-you know you’re going to have time in between and so I did a lot of walking and they told me when I checked into that hospital that 11 times around the out- the per-perimeter was a mile. So I set goals, I’m gonna do my exercise.
John: There were a few days where I had trouble getting up and ah I did walk around but I didn’t record… I mentioned that I didn’t do any laps. What I call laps are when I was really moving… and ah I did get up and walk around very slowly and… those days I didn’t do much. But I had to try and do something.
John: My advice for somebody going through this is have the support, have a positive positive attitude, keep in shape, the best shape you can be.
John: Ah We - we look at it like it’s a new day every day.