Real-world data: MRD
Real-world studies of VYXEOS examined rates of MRD negativity,2-4 a marker of deep remission5
MRD was not assessed in the Phase 3 study for VYXEOS, and its relevance as a predictor of OS remains a topic for further investigation.6
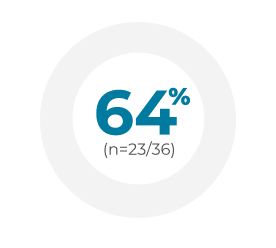
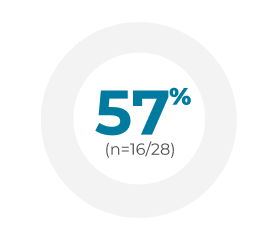
In real-world studies, up to 64% of patients treated with VYXEOS achieved MRD negativity following CR/CRi.2-4
MRD assessment may occur after the completion of initial induction and before HSCT.7
-
 Germany2
Germany2Retrospective chart review (N=188) of newly diagnosed patients median age 65 years (range: 26-89) with AML-MRC or t-AML treated with VYXEOS across 25 German centers between 2018 and 2020. Dosing was administered per the EU label.a Patient characteristics were similar to the Phase 3 study, but the treatment course substantially differed in that a much lower frequency (14%) received second induction with VYXEOS compared with 31% in the Phase 3 study. Of patients who achieved CR/CRi (n=85) after induction, 36 were tested for MRD at the time of transplant.
Safety (n=188) was consistent with the Phase 3 study. Day 30 mortality after VYXEOS induction (n=176) was 8% (60-day mortality was not reported). Grade 3/4 nonhematologic toxicities included infection (22%), pneumonia (22%), febrile neutropenia (15%), gastrointestinal (4%), bleeding (4%), and renal failure (3%). In patients with CR/CRi, recovery to ANC ≥500/μl and platelet count ≥50,000/μl was observed in 95% and 92% of patients, respectively. Median time to ANC and platelet recovery was 33 days (range: 6-99 days) and 30 days (range: 7-77 days), respectively. -
 France3
France3Retrospective chart review (N=103) of newly diagnosed, previously untreated adult patients diagnosed with AML-MRC or t-AML across 12 French centers treated with VYXEOS between April 2018 and November 2019. Patients received 1 or 2 induction cycles. There was one exception to the Phase 3 study: eligible patients were aged >18 years; however, the median age was 67 years (range: 20-83). Dosing was administered per the US/EU labels.a Baseline patient characteristics were similar to the Phase 3 study, with the exception of prior HMA exposure (18% vs 40.5% in the VYXEOS arm of the Phase 3 study) and age range (>18 years vs 60-75 years in the Phase 3 study). Of patients who achieved CR/CRi (n=61), 28 were tested for MRD at the time of first consolidation cycle.
Safety (n=103) was consistent with the Phase 3 study. 30-day mortality was 6% and 60-day mortality was 8%. Adverse events during treatment regardless of causality reported as all grades and with a frequency of ≥10% included febrile neutropenia (91%), gastrointestinal toxicity (50%), pneumonia (36%), nausea (36%), rash (25%), mucositis (22%), vomiting (13%), bleeding (12%), alopecia (11%), and hypertensive crisis (10%). Neutrophil count and platelet count remained >0.5 x 109/L and 20 x 109L in only 1 patient and 2 patients, respectively. Median time to neutrophil recovery (>0.5 x 109/L) and platelet recovery (>20 x 109L) after the first induction was 29 days (range: 19-146 days) and 28 days (range: 12-77 days), respectively. Nine patients discontinued treatment due to prolonged hematological toxicity. -
 Italy4
Italy4Retrospective chart review (N=71) across 31 centers in the Italian Compassionate Use Program for VYXEOS between June 2018 and July 2019. Patients median age 66 years (range: 52-79) had confirmed sAML or t-AML according to WHO 2016 criteria. Dosing was administered per the Phase 3 study.a Six patients (9%) presented with ECOG PS 3-4 upon enrollment. Among patients who achieved CR/CRi after first induction (n=46), 40 patients were assessed with MFC-MRD and 38 were tested with WT-1 based MRD assessment.
Safety (n=71) was consistent with the Phase 3 study. 30-day mortality was 4.2% (n=3/71) and 60-day mortality was 7% (n=5/71). 80.3% of patients experienced > grade 1 adverse events during induction. Most adverse events were infections, including fever of unknown origin (28%), sepsis (28%), and pneumonia (11.3%). Others adverse events included mucositis (7%) and a self-resolving diffuse skin rash (25.4%). Median time to neutrophil recovery (>0.5 x109/L) and platelet recovery (>25 x109/L) was 38 days (range: 12-60 days) and 28 days (range: 12-60 days), respectively.
Limitations
- Results cannot be compared across studies as MRD assessment in AML lacks standardization with several technologies available for MRD assessment5
- Results should be interpreted with caution as these are real-world evidence studies that are not powered to determine statistical significance
- Direct comparisons between real-world evidence studies and randomized controlled trials are difficult8
- ANC=absolute neutrophil count;
MRD=minimal/measurable residual disease;
t-AML=therapy-related acute myeloid leukemia;
WHO=World Health Organization;
WT1=Wilms tumor 1. - aDosing regimens in the US and EU labels are identical.9,10
Efficacy
VYXEOS is the only FDA-approved intensive chemotherapy that delivers on key treatment milestones needed to beat sAML better than 7+3.6,10 Learn more about lasting remission, increased chance of HSCT, and prolonged survival.
more
About sAML
Secondary AML is a broad category of high-risk AML that includes multiple subtypes of leukemias with different characteristics but similar poor prognosis and significant challenge to reach a cure.11,12 Learn more about which patients may be appropriate for intensive chemotherapy with VYXEOS.
more


