Rethink the potential of your patients
Know the seriousness
of
their sAML
diagnosis
sAML is a broad category of high-risk AML that includes multiple subtypes of leukemias with different characteristics but similar poor prognosis and significant challenge to reach a cure.1,2

SUBTYPES OF sAML
sAML includes multiple subtypes of high-risk AML1,2
As of 2016, sAML includes therapy-related AML (t-AML), AML with antecedent hematologic disease (AHD), and de novo AML with myelodysplastic related changes (MRC).1
t-AML accounts for up to 10% of all AML cases and is defined as AML arising after exposure to chemotherapy or radiotherapy for previous malignancies.1,3
Prior malignancies
associated with t-AML4,a
- 3%Acute lymphoblastic leukemia
- 4%Multiple myeloma
- 10%Rheumatic disease
- 32%Lymphoproliferative disorder
- 51%Solid cancer
aDistribution of previous disease in 203 patients with t-AML regardless of treatment intent. Data from a national, population-based study of 3055 patients diagnosed with AML from 2000 to 2013 in Denmark.4
Prior malignancies associated with t-AML4,a

aDistribution of previous disease in 203 patients with t-AML regardless of treatment intent. Data from a national, population-based study of 3055 patients diagnosed with AML from 2000 to 2013 in Denmark.4
AML-MRC accounts for up to 35% of all AML cases,5,b but can still be difficult to identify because of its multifaceted nature1,2
The 2016 WHO classification defines AML-MRC as ≥20% blasts in the peripheral blood or bone marrow and ANY of the following6,7:
MDS
Previously documented
MDS or MDS/MPN
Non-MDS
Myelodysplasia-related
cytogenetic
abnormalitiesc-e
Multilineage dysplasia: Dysplasia present in ≥50% of
cells in
at least
2 myeloid cell lines, unless an NPM1
mutation or
biallelic mutation of CEBPA is present
A majority of AML-MRC cases DO NOT present with a documented history of MDS or MDS/MPN.8,f
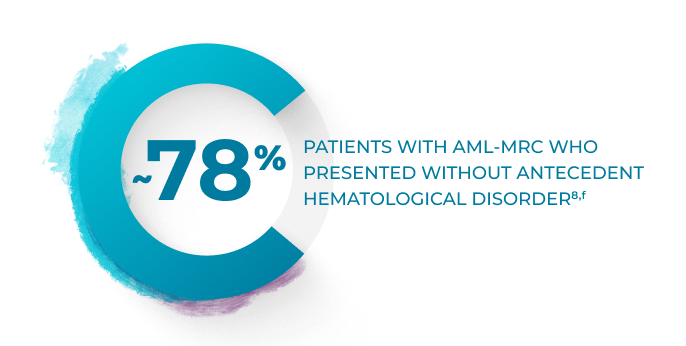
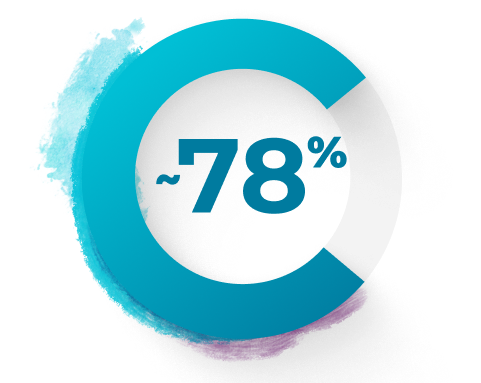
PATIENTS WITH AML-MRC WHO PRESENTED WITHOUT ANTECEDENT HEMATOLOGICAL DISORDER8,f
As AML-MRC often occurs as de novo AML with MDS-related
cytogenetic changes or multilineage dysplasia, patients
with
AML-MRC may go unidentified without comprehensive
testing.9
Genetic testing for driver mutations of AML can help identify patients with sAML who have greater need for intensive treatment.10
- Patients with mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, and STAG2 genes are at risk of evolution from MDS into AML2
- 78% of patients with sAML present with pan-AML mutations, such as those of myeloid transcription factors (RUNX1, CEBPA, GATA2) and signal transduction proteins (FLT3 or RAS pathway)2
- 15% of sAML cases present with mutations in the TP53 gene, characterized by poor prognosis2
Fully identifying the AML subtype is important to choosing
treatment,
and studies have shown delaying
treatment for genetic and laboratory testing does not negatively
impact outcomes.11,12,g
CEBPA=CCAAT/enhancer-binding protein-alpha; ECOG=Eastern Cooperative Oncology Group Performance Status; IC=intensive chemotherapy; MDS=myelodysplastic syndromes; MPN=myeloproliferative neoplasm; NPM1=nucleophosmin-1; SAL=Study Alliance Leukemia; WHO=World Health Organization.
bIncidence is variable based on the definition of AML-MRC used.5
cComplex karyotype: 3 or more abnormalities.7
dUnbalanced abnormalities: -7/del(7q), del(5q)/t(5q), i(17q)/t(17p), -13/del(13q), del(11q), del(12p)/t(12p), idic(X)(q13).7
eBalanced abnormalities: t(11;16)(q23.3;p13.3), t(3:21)(q26.2;q22.1), t(1;3)(p36.3;q21.2), t(2;11)(p21;q23.3), t(5;12)(q32;p13.2), t(5;7)(q32;q11.2), t(5;17)(q32;p13.2), t(5;10)(q32;q21.2), t(3;5)(q25.3;q35.1).7
fStudy of 175 adult patients with AML-MRC classified by 2008 WHO criteria.8
gTwo studies looked at the effect of time from diagnosis to treatment start on patient prognosis: results from a retrospective study in patients with de novo AML or sAML aged ≥60 years (n=664)12 and an analysis of real-world SAL-AML registry data of patients with newly diagnosed AML, including sAML (n=2263).11
Efficacy
VYXEOS is the only FDA-approved intensive chemotherapy that delivers on key treatment milestones needed to beat sAML better than 7+3.13,14 Learn more about lasting remission, increased chance of HSCT, and prolonged survival.
more
Safety
The safety profile of VYXEOS in the Phase 3 study was comparable with 7+3, with similar types and severity of adverse reactions.15
more
INDICATION

VYXEOS is indicated for the treatment of newly-diagnosed therapy-related acute myeloid leukemia (t‑AML) or AML with myelodysplasia-related changes (AML-MRC) in adults and pediatric patients 1 year and older.
Important Safety Information


WARNING: DO NOT INTERCHANGE WITH OTHER DAUNORUBICIN AND/OR CYTARABINE-CONTAINING PRODUCTS
VYXEOS has different dosage recommendations than daunorubicin hydrochloride injection, cytarabine injection, daunorubicin citrate liposome injection, and cytarabine liposome injection. Verify drug name and dose prior to preparation and administration to avoid dosing errors.
Contraindications
VYXEOS is contraindicated in patients with a history of serious hypersensitivity reactions to cytarabine, daunorubicin, or any component of the formulation.
Warnings and Precautions
Hemorrhage
Serious or fatal hemorrhage events, including fatal CNS hemorrhages, associated with prolonged thrombocytopenia, have occurred with VYXEOS. The overall incidence (grade 1-5) of hemorrhagic events was 74% in the VYXEOS arm and 56% in the control arm. The most frequently reported hemorrhagic event was epistaxis (36% in VYXEOS arm and 18% in control arm). Grade 3 or greater events occurred in 12% of VYXEOS-treated patients and in 8% of patients in the control arm. Fatal treatment-emergent CNS hemorrhage not in the setting of progressive disease occurred in 2% of patients in the VYXEOS arm and in 0.7% of patients in the control arm. Monitor blood counts regularly and administer platelet transfusion support as required.
Cardiotoxicity
VYXEOS contains daunorubicin, which has a known risk of cardiotoxicity. This risk may be increased in patients with prior anthracycline therapy, preexisting cardiac disease, previous radiotherapy to the mediastinum, or concomitant use of cardiotoxic drugs. Assess cardiac function prior to VYXEOS treatment and repeat prior to consolidation and as clinically required. Discontinue VYXEOS in patients with impaired cardiac function unless the benefit of initiating or continuing treatment outweighs the risk. VYXEOS is not recommended in patients with cardiac function that is less than normal.
Total cumulative doses of non-liposomal daunorubicin greater
than
550 mg/m2 have been associated with an increased
incidence of
drug-induced congestive heart failure. The tolerable limit
appears
lower
(400 mg/m2) in patients who
received radiation therapy to
the
mediastinum. Calculate the lifetime cumulative anthracycline
exposure prior to each cycle of VYXEOS. VYXEOS is not
recommended in
patients whose lifetime anthracycline exposure has reached the
maximum cumulative limit.
Hypersensitivity Reactions
Serious or fatal hypersensitivity reactions, including anaphylactic reactions, have been reported with daunorubicin and cytarabine. Monitor patients for hypersensitivity reactions. If a mild or moderate hypersensitivity reaction occurs, interrupt or slow the rate of infusion with VYXEOS and manage symptoms. If a severe or life-threatening hypersensitivity reaction occurs, discontinue VYXEOS permanently, treat the symptoms, and monitor until symptoms resolve.
Copper Overload
VYXEOS contains copper. Consult with a hepatologist and nephrologist with expertise in managing acute copper toxicity in patients with Wilson’s disease treated with VYXEOS. Monitor total serum copper, serum non-ceruloplasmin-bound copper, 24-hour urine copper levels, and serial neuropsychological examinations during VYXEOS treatment in patients with Wilson’s disease or other copper-related metabolic disorders. Use only if the benefits outweigh the risks. Discontinue in patients with signs or symptoms of acute copper toxicity.
Tissue Necrosis
Daunorubicin has been associated with severe local tissue necrosis at the site of drug extravasation. Administer VYXEOS by the intravenous route only. Confirm patency of intravenous access before administration. Do not administer by intramuscular or subcutaneous route.
Embryo-Fetal Toxicity
VYXEOS can cause embryo-fetal harm when administered to a pregnant woman. Patients should avoid becoming pregnant while taking VYXEOS. If VYXEOS is used during pregnancy or if the patient becomes pregnant while taking VYXEOS, apprise the patient of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of VYXEOS.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions (incidence ≥25%) are hemorrhagic events (74%), febrile neutropenia (70%), rash (56%), edema (55%), nausea (49%), mucositis (48%), diarrhea (48%), constipation (42%), musculoskeletal pain (43%), fatigue (39%), abdominal pain (36%), dyspnea (36%), headache (35%), cough (35%), decreased appetite (33%), arrhythmia (31%), pneumonia (31%), bacteremia (29%), chills (27%), sleep disorders (26%), and vomiting (25%).
Please see full Prescribing Information, including BOXED Warning.
References: 1. Lalayanni C, Gavriilaki E, Athanasiadou A, et al. Secondary acute myeloid leukemia (sAML): similarly dismal outcomes of AML after an antecedent hematologic disorder and therapy related AML. Clin Lymphoma Myeloma Leuk. 2022;22(4):e233-e240.
2. Capelli D, Menotti D, Florentini A, Sarcani F, Olivieri A. Chapter 7: Secondary acute myeloid leukemia: pathogenesis and treatment. Accessed March 21, 2024. https://www.ncbi.nlm.nih.gov/books/NBK586211/?report=printable
3. Leone G, Mele L, Pulsoni A, et al. The incidence of secondary leukemias. Haematologica. 1999;84(10):937-945.
4. Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER hematopoietic and lymphoid neoplasm database: acute myeloid leukemia with myelodysplasia-related changes. Accessed March 21, 2024. https://seer.cancer.gov/seertools/hemelymph/
51f6cf58e3e27c3994bd53ae/?q=aml-mrc
6. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937‐951.
7. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391‐2405.
8. Miesner M, Haferlach C, Bacher U, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood. 2010;116(15):2742-2751.
9. Arber DA, Erba HP. Diagnosis and treatment of patients with acute myeloid leukemia with myelodysplasia-related changes (AML-MRC). Am J Clin Pathol. 2020;154(6):731-741.
10. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed April 3, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org.
11. Röllig C, Kramer M, Schliemann C, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136(7):823-830.
12. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
13. VYXEOS [package insert]. Palo Alto, CA: Jazz Pharmaceuticals.
14. Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491.
15. Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692.
