INDICATION 
VYXEOS is indicated for the treatment of newly-diagnosed
therapy-related acute myeloid leukemia (t‑AML) or AML with
myelodysplasia-related changes (AML-MRC) in adults and pediatric
patients 1 year and older.
WARNING: DO NOT INTERCHANGE WITH OTHER DAUNORUBICIN AND/OR
CYTARABINE-CONTAINING PRODUCTS
VYXEOS has different dosage recommendations than
daunorubicin
hydrochloride injection, cytarabine injection, daunorubicin
citrate liposome injection, and cytarabine liposome
injection.
Verify drug name and dose prior to preparation and
administration
to avoid dosing errors.
Contraindications
VYXEOS is contraindicated in patients with a history of serious
hypersensitivity reactions to cytarabine, daunorubicin, or any
component of the formulation.
Warnings and Precautions
Hemorrhage
Serious or fatal hemorrhage events, including fatal CNS hemorrhages,
associated with prolonged thrombocytopenia, have occurred with
VYXEOS. The overall incidence (grade 1-5) of hemorrhagic events was
74% in the VYXEOS arm and 56% in the control arm. The most
frequently reported hemorrhagic event was epistaxis (36% in VYXEOS
arm and 18% in control arm). Grade 3 or greater events occurred in
12% of VYXEOS-treated patients and in 8% of patients in the control
arm. Fatal treatment-emergent CNS hemorrhage not in the setting of
progressive disease occurred in 2% of patients in the VYXEOS arm and
in 0.7% of patients in the control arm. Monitor blood counts
regularly and administer platelet transfusion support as required.
Cardiotoxicity
VYXEOS contains daunorubicin, which has a known risk of
cardiotoxicity. This risk may be increased in patients with prior
anthracycline therapy, preexisting cardiac disease, previous
radiotherapy to the mediastinum, or concomitant use of cardiotoxic
drugs. Assess cardiac function prior to VYXEOS treatment and repeat
prior to consolidation and as clinically required. Discontinue
VYXEOS in patients with impaired cardiac function unless the benefit
of initiating or continuing treatment outweighs the risk. VYXEOS is
not recommended in patients with cardiac function that is less than
normal.
Total cumulative doses of non-liposomal daunorubicin greater than
550 mg/m2 have been associated with an increased
incidence of
drug-induced congestive heart failure. The tolerable limit appears
lower
(400 mg/m2) in patients who received radiation
therapy to the
mediastinum. Calculate the lifetime cumulative anthracycline
exposure prior to each cycle of VYXEOS. VYXEOS is not recommended in
patients whose lifetime anthracycline exposure has reached the
maximum cumulative limit.
Hypersensitivity Reactions
Serious or fatal hypersensitivity reactions, including anaphylactic
reactions, have been reported with daunorubicin and cytarabine.
Monitor patients for hypersensitivity reactions. If a mild or
moderate hypersensitivity reaction occurs, interrupt or slow the
rate of infusion with VYXEOS and manage symptoms. If a severe or
life-threatening hypersensitivity reaction occurs, discontinue
VYXEOS permanently, treat the symptoms, and monitor until symptoms
resolve.
Copper Overload
VYXEOS contains copper. Consult with a hepatologist and nephrologist
with expertise in managing acute copper toxicity in patients with
Wilson’s disease treated with VYXEOS. Monitor total serum copper,
serum non-ceruloplasmin-bound copper, 24-hour urine copper levels,
and serial neuropsychological examinations during VYXEOS treatment
in patients with Wilson’s disease or other copper-related metabolic
disorders. Use only if the benefits outweigh the risks. Discontinue
in patients with signs or symptoms of acute copper toxicity.
Tissue Necrosis
Daunorubicin has been associated with severe local tissue necrosis
at the site of drug extravasation. Administer VYXEOS by the
intravenous route only. Confirm patency of intravenous access before
administration. Do not administer by intramuscular or subcutaneous
route.
Embryo-Fetal Toxicity
VYXEOS can cause embryo-fetal harm when administered to a pregnant
woman. Patients should avoid becoming pregnant while taking VYXEOS.
If VYXEOS is used during pregnancy or if the patient becomes
pregnant while taking VYXEOS, apprise the patient of the potential
risk to a fetus. Advise females and males of reproductive potential
to use effective contraception during treatment and for 6 months
following the last dose of VYXEOS.
MOST COMMON ADVERSE REACTIONS
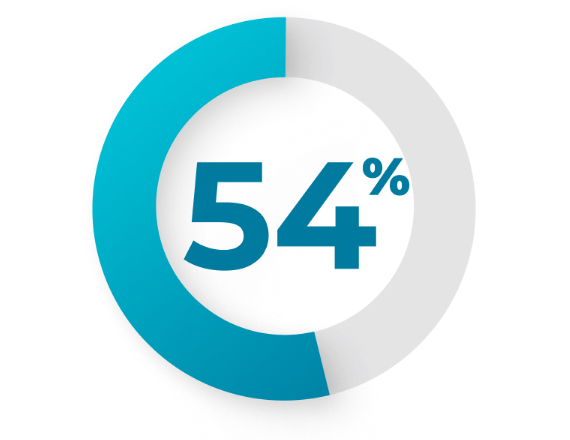
The most common adverse reactions (incidence ≥25%) are hemorrhagic
events (74%), febrile neutropenia (70%), rash (56%), edema (55%),
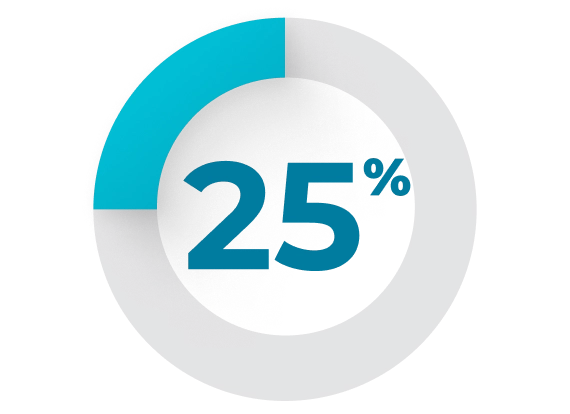
nausea (49%), mucositis (48%), diarrhea (48%), constipation (42%),
musculoskeletal pain (43%), fatigue (39%), abdominal pain (36%),
dyspnea (36%), headache (35%), cough (35%), decreased appetite
(33%), arrhythmia (31%), pneumonia (31%), bacteremia (29%), chills
(27%), sleep disorders (26%), and vomiting (25%).
Please see full Prescribing Information,
including
BOXED Warning.